Press Release
Amnovis produces more cost-effective titanium spinal cages through significant Additive Manufacturing productivity increase
Aarschot, Belgium – October 6, 2022.
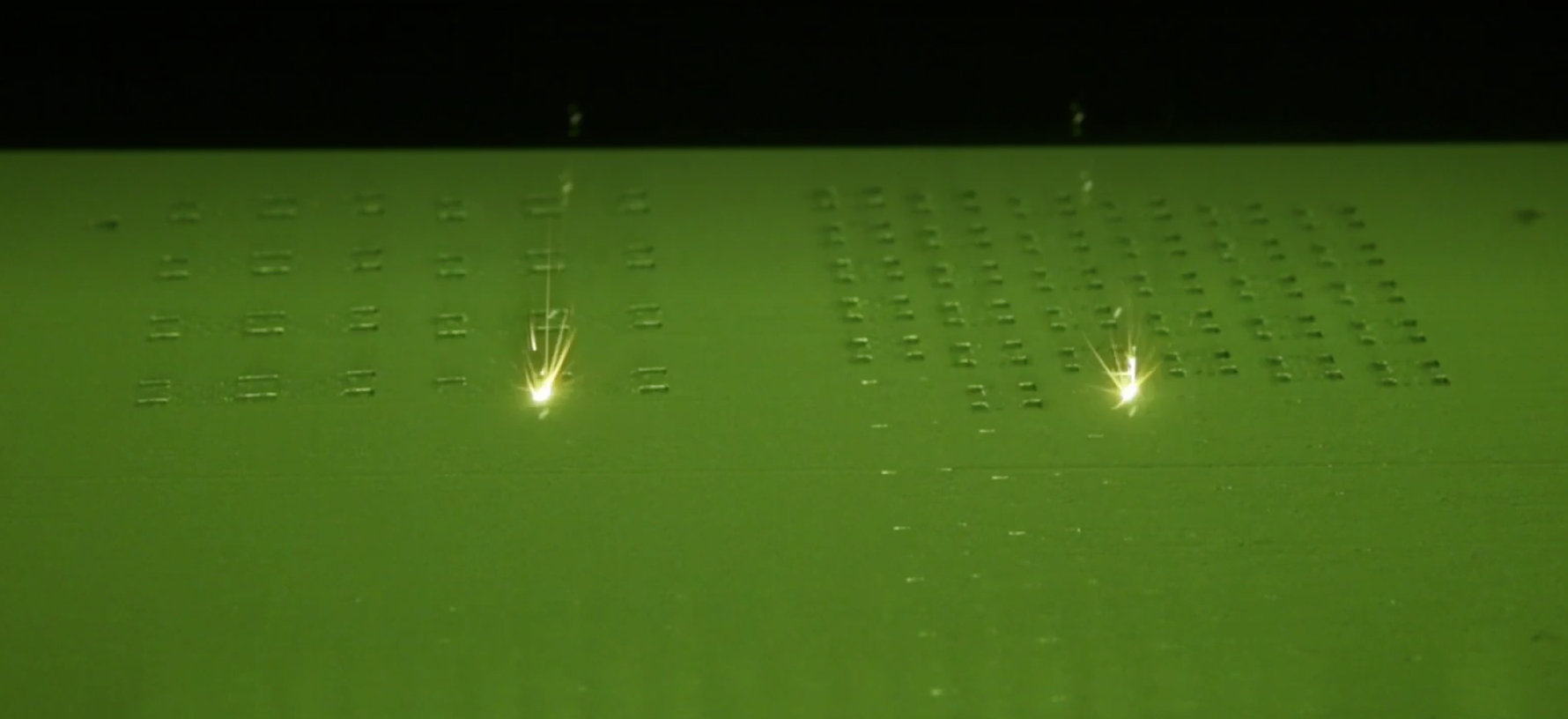
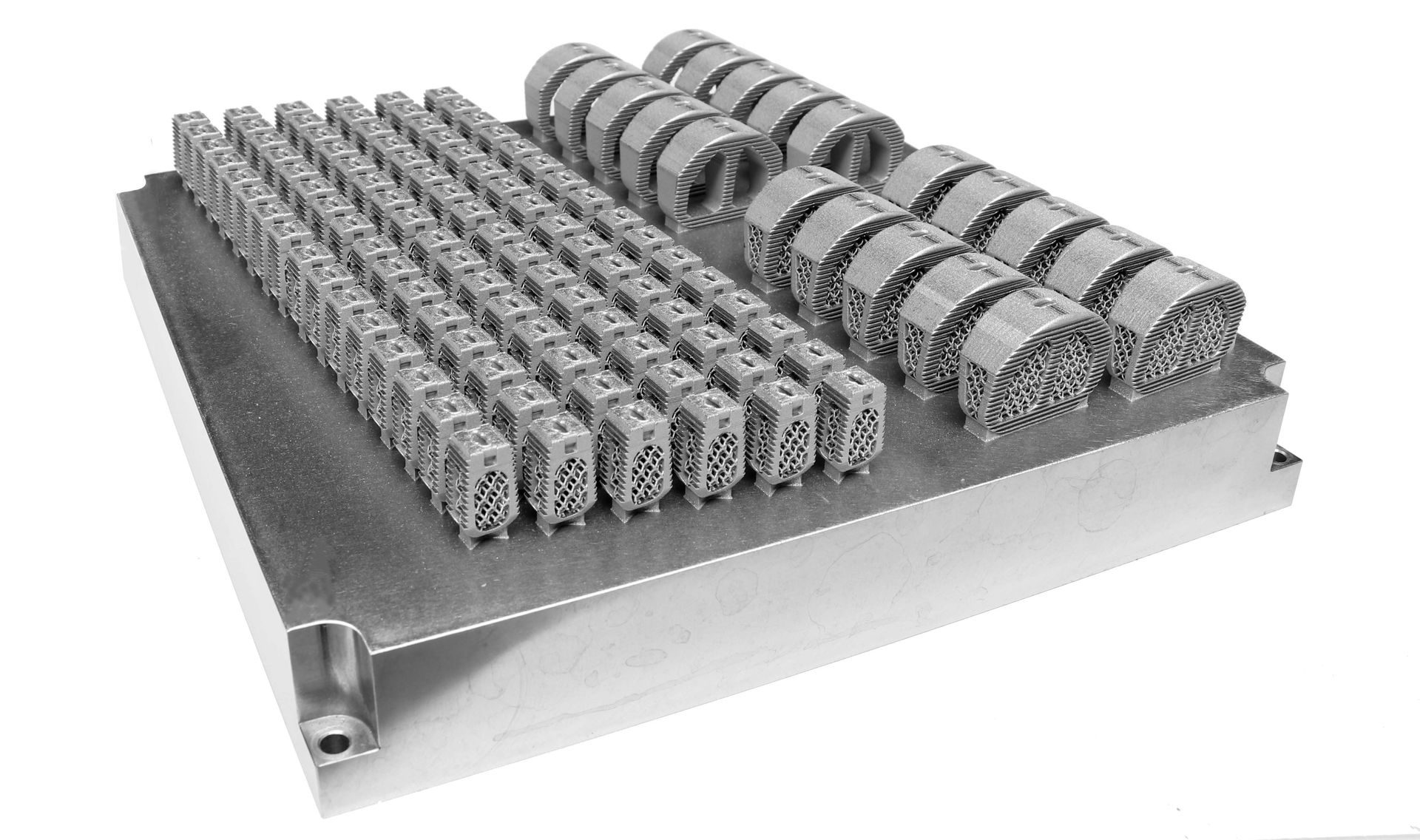
Amnovis has incorporated process improvements into its AM contract manufacturing workflow for titanium implants. This leads to significantly increased productivity, which is good news for titanium spinal cage and other medical device OEMs. Amnovis is now able to produce 3D printed spinal cages at lower cost and shorter lead times. Visit the Amnovis booth (#2627) next week at NASS Annual Meeting in Chicago where these 3D printed devices will be on display.
Ruben Wauthle, Amnovis CEO and Co-Founder: "For us, innovation benefits from material and process enhancements as well as the ability to increase AM productivity. By scaling our manufacturing capabilities for quality-critical applications, such as medical devices, we ensure faster delivery at lower cost, while maintaining our quality standards.”
Driven by the track traveled so far, Amnovis decided to shift gears to further strengthen its position in the rapidly evolving medical market. The Amnovis’ founders are AM pioneers who were among the first to employ laser powder bed fusion (LPBF) for printing titanium medical implants. Incorporating the process improvements into its manufacturing workflow allow Amnovis to expand its potential for standard and patient-specific medical device innovation.
Ruben Wauthle: “We are happy to share our competitive AM capabilities at NASS 2022 Annual Meeting in Chicago and discuss manufacturing applications for spinal cages and other implants.”
"We see great potential in innovating various AM applications across a broad spectrum of medical device types. Highly-efficient 3D printers enable us to better align design and development with material selection and manufacturing excellence. Our joint offering with selected partners comprises of the complete process for customers and prospects to aim high and move fast, while removing hurdles in medical device development, validation and manufacturing."
“At Amnovis, we rely on our comprehensive AM workflow and production platform, which is entirely ISO 13485:2016 certified. Digital process automation enables us to provide full traceability and repeatability to flexibly scale up manufacturing of high-end products for quality-critical medical applications. Overall, we are maximizing productivity, reliability, uptime and yield in our efforts to better serve titanium spinal cage and other medical device OEMs."
About Amnovis
Founded by Peter Mercelis and Ruben Wauthle and based in Aarschot, Belgium, Amnovis is a manufacturing and engineering company that uses innovative additive manufacturing (AM) technologies and materials for high-end applications such as medical devices. As an ISO 13485 certified contract manufacturer, Amnovis offers best-in-class AM technologies and expertise and paves the way for emerging AM technologies and materials. Digital process automation provides the traceability and repeatability to flexibly scale up manufacturing of products that comply with customer and regulatory requirements.
For more information, visit www.amnovis.com or follow Amnovis on LinkedIn.
Contact us for more high-resolution pictures.
DOWNLOADS